סמינר מיוחד בכימיה אורגנית: Towards the design of effective antibacterial agents
Dr. Zvi Hayouka, Chemistry department, University of Wisconsin-Madison
Towards the design of effective antibacterial agents
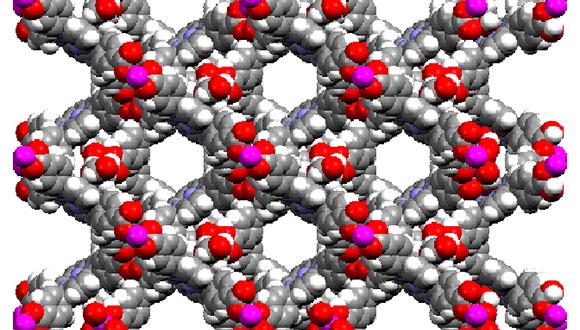
Pathogenic infections represent a persistent threat to human health. The rapid development of resistance to drug therapies creates a continuing need for developing new anti-infective agents. Host-Defense Peptides (HDPs) represent a potential source of inspiration for development of new antibacterial agents. Elucidation of high-resolution structure has been challenging for the large group of HDPs. We used racemic crystallization to obtain the crystal structure of an analogue of the widely-studied HDP, magainin 2. The crystal structure features a dimerization mode that has previously been proposed to play a role in the antibacterial activity of magainin 2 and related peptides (1).
The broad molecular diversity among HDPs suggests that their prokaryotic-selective activity is not tightly coupled to specific features of amino acid sequence or peptide conformation. This situation has inspired the development of several families of sequence-random hydrophobic-cationic co-polymers that display antibacterial behavior with varying levels of hemolytic activity. We employed solid-phase synthesis in an unconventional way to generate peptide mixtures that contain one type of hydrophobic residue and one type of cationic residue. Each mixture was random in terms of sequence, but highly controlled in terms of chain length and stereochemistry. Analysis of the antibacterial and hemolytic properties of these mixtures revealed that selective antibacterial activity can be achieved with heterochiral binary mixtures but not homochiral binary mixtures (2).
We have explored nylon-3 materials (poly-b-peptides) as functional mimics of HDPs (3, 4). Nylon-3 polymers are readily synthesized via ring-opening polymerization of b-lactams; some hydrophobic-cationic copolymers display broad antimicrobial activity. It is challenging to perform structure-activity relationships studies for nylon-3 copolymers (or other copolymers) because each sample contains many different kinds of molecules (different lengths, different subunit compositions, different subunit sequences, different subunits stereochemistries). Using our unconventional solid-phase approach we were able to prepare nylon-3 copolymer mixtures that are better defined than are the mixtures generated via ring-opening polymerization of b-lactams. This synthetic strategy is not available for any other types of polymers that have been reported to function as selective antibacterial agents. Our findings show that chain length and stereochemistry are important parameters in terms of determining the activity of polymers of this type.
1. Hayouka, Z. Chakraborty, S. Liu, R. Gellman, H.S. (2013). Interplay Among Subunit Identity, Composition, Chain Length and Stereochemistry in the Activity Profile of Sequence-Random Oligomer Mixtures. J. Am. Chem. Soc. 135(32):11748-5.
2. Hayouka, Z. Mortenson, D.E. Kreitler, D.F. Weisblum, B. Forest, K.T. Gellman, H.S. (2013). Evidence for Phenylalanine Zipper-Mediated Dimerization in the X-Ray Crystal Structure of a Magainin 2 Derivative. J. Am. Chem. Soc. 135(42):15738-41.
3. Liu, R. Chakraborty, S. Hayouka, Z. Gellman, H.S. (2013). Nylon-3 polymer as antifungal agent. J. Am. Chem. Soc. 135, 5270-3.
4. Chakraborty, S. Liu, R. Hayouka, Z. Gellman. H.S. (2013). Studying the effect of cyclic versus acyclic nylon-3 polymers. ACS Macro Letters, 2013, 2, 753.